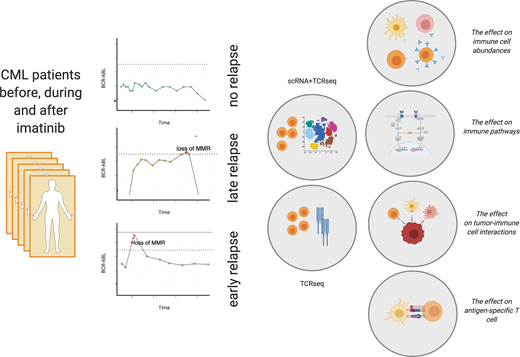
Only half of chronic myeloid leukemia (CML) patients in deep molecular remission are able to maintain treatment free remission (TFR) after tyrosine kinase inhibitor (TKI) discontinuation. Identifying predictive markers for TFR remains a key issue. The immunological control or eradication of TKI insensitive leukemic stem cells may contribute to successful cessation, as recent work has demonstrated that differences in immune cell compositions can be associated with better probability of TFR.
In order to understand the precise immunological changes in CML, we profiled over 170,000 cells from 25 CML samples from different clinical phases (untreated, during TKI therapy and after TKI cessation) using single-cell RNA and T cell receptor (TCR) αβ sequencing (scRNA+TCRab-seq). We profiled both CD45+ sorted peripheral blood samples (n=20) and CD34+ sorted bone marrow samples (n=5). To understand antigen-specific responses, we profiled TCRs specific to tumor-antigen PR1 (n=12) and compared these to unsorted TCRβ-sequenced samples from CML (n=137) and healthy donors (n=786).
To understand the distinctive immunological features in CML, we compared the immune system in CML (n=20) to those in other hematological and solid malignancies profiled with scRNA+TCRab-seq (n=9). We discovered NK cells to be the most unique feature in CML, as NK CD56dim cells were more abundant in CML patients (Padj<0.001) and the NK cell repertoire was more transcriptionally heterogenous and included otherwise rarely seen exhausted NK population with upregulated HAVCR2 and TIGIT expression (Padj <0.0001). Further, the NK cell maturation changed during TKI-treatment from an effector CD56dim state towards an adaptive NK-cell state.
We sought to understand whether the immunological activity differs before and after TKI discontinuation in different clinical outcomes following discontinuation. Therefore, we reclustered the discontinuation samples from three different outcomes: TFR, early relapse (<6 months after cessation) or late relapse (>6 months). In all clinical outcomes, TKI cessation invigorated NK cell exhaustion and the most upregulated pathways included IFNg and TNFa via NFkB pathways. Patients with successful TFR had less differences in their immune system after cessation in comparison to relapsing patients, which could hint that their immune subsets were better prepared for the cessation. Unlike the early relapse patients' quiescent immune cells, the late relapse patients' immune subsets were active, but under pronounced inhibitory Treg signals, providing a clinically interesting approach to alter the outcome of these patients.
Next, to understand how malignant CML cells evade or interact with the immune system, we analyzed CD34+ cells and calculated a BCR-ABL1 activity score. The most primitive CD34+ cells had lower BCR-ABL1 score and not as many immunological ligand-receptor pairs as their less primitive, highly BCR-ABL1 pathway expressing, counterparts (P<0.0001). LGALS9, that is expressed on both BCR-ABL1 high and low CD34+ cells, arose as one of the most interesting ligands mediating tumor-immune cell interactions and it has many potential receptors expressed by NK cells, including HAVCR2.
Finally, we found that tumor-antigen PR1 is preferentially expressed on CD34+ cells with high BCR-ABL1 score. Most TCRs recognizing PR1 are restricted to individuals, but they share short amino-acid motifs that are shared across patients. Thus, we were able to generate an in silico tool to recognize PR1-specific TCRs from unsorted TCR-seq samples with high accuracy (AUC 0.91 from 10-fold cross validation). With our tool, we were able to show that anti-PR1 responses were more frequent in CML than in healthy donors (P<0.0001), are more expanded in bone-marrow than in blood (P<0.0001) and diversified during TKI treatment (P<0.0001). In the scRNA+TCRab-seq data, the anti-PR1 response differed between TFR and early relapse patient significantly at phenotype level, where the TFR patient had cytotoxic anti-leukemic response before TKI cessation, in comparison to the early relapse patient's exhausted and less cytotoxic PR1-specific cells.
In conclusion, our results provide high-resolution insights into anti-leukemic immune responses in CML and how we could harness and monitor them to enable successful TKI cessations.
Olsson-Strömberg:Pfizer: Research Funding. Hjorth-Hansen:Pfizer: Honoraria, Research Funding; Austrian Orphan Pharma: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding. Mustjoki:Pfizer: Research Funding; Novartis: Research Funding; BMS: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.